The Discovery Of Electron.
Introduction:
The discovery of the electron marked a pivotal moment in the history of science. It laid the foundation for modern physics and revolutionized our understanding of the fundamental building blocks of matter. This remarkable journey of discovery spanned several decades and involved the contributions of numerous scientists, each making their own invaluable contributions to unraveling the mysteries of the electron. In this comprehensive account, we delve into the story of the electron’s discovery, highlighting key milestones and the scientists who played critical roles in this groundbreaking journey.
I. Background: Early Theories of Electricity and Matter
To understand the context in which the discovery of the electron took place, we must first explore the early theories of electricity and matter. Throughout history, philosophers and scientists speculated on the nature of matter and electricity. From the ancient Greeks’ notion of fundamental elements to the experiments of early modern scientists, such as William Gilbert and Robert Hooke, the stage was set for deeper investigations into the fundamental properties of matter.
II. The Beginnings of Electric Current and Cathode Rays
The 18th and 19th centuries saw significant advancements in the study of electricity and the behavior of gases in low-pressure conditions. Scientists like Luigi Galvani, Alessandro Volta, and Michael Faraday laid the groundwork for understanding electrical currents and their effects on matter. In the early 19th century, Sir William Crookes conducted experiments using cathode ray tubes, which led to the discovery of cathode rays and their peculiar behavior.
III. The Work of J.J. Thomson
One of the most influential figures in the discovery of the electron was Joseph John Thomson, commonly known as J.J. Thomson. In the late 19th century, Thomson conducted a series of experiments with cathode ray tubes and made groundbreaking observations. Through meticulous experimentation and analysis, he deduced the existence of a negatively charged particle smaller than an atom – the electron. Thomson’s groundbreaking work earned him the Nobel Prize in Physics in 1906.
IV. The Millikan Oil Drop Experiment
While Thomson’s work provided strong evidence for the existence of electrons, the precise charge and mass of the electron remained unknown. Enter Robert A. Millikan, an American physicist who devised the famous “Oil Drop Experiment.” In this experiment, Millikan measured the charge of individual oil droplets, which helped determine the charge of the electron and, subsequently, its mass. His work earned him the Nobel Prize in Physics in 1923.
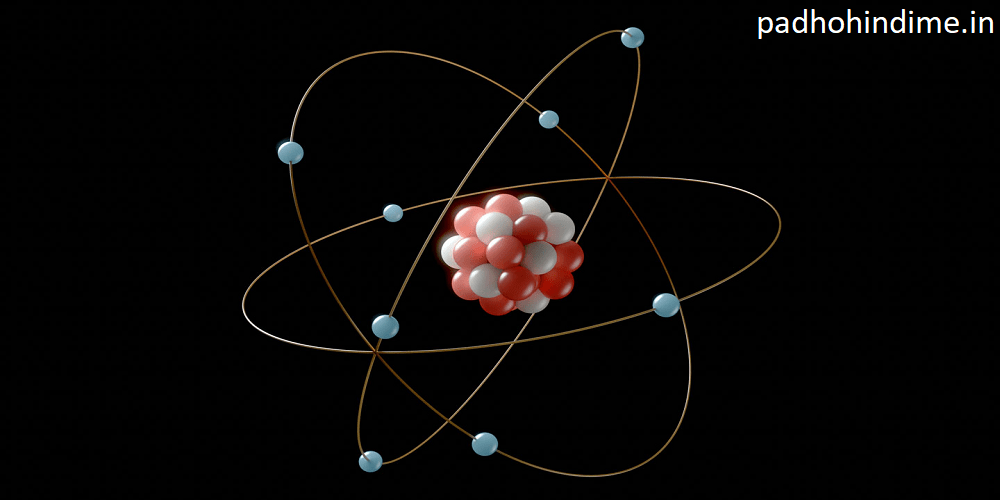
V. The Electron’s Role in Atomic Structure
The discovery of the electron fundamentally altered our understanding of atomic structure. With the identification of a subatomic particle carrying a negative charge, scientists began to develop models of the atom that incorporated electrons orbiting a positively charged nucleus. This led to the development of Rutherford’s nuclear model and later to Bohr’s model, which paved the way for quantum mechanics and the modern atomic theory.
VI. The Role of Electrons in Chemical Bonding
As researchers delved deeper into the behavior of electrons, they discovered that these tiny particles play a crucial role in chemical bonding. Gilbert N. Lewis, Irving Langmuir, and Linus Pauling made significant contributions to our understanding of electron configurations and how they govern chemical interactions. Their work laid the foundation for the field of quantum chemistry.
VII. Advancements in Particle Physics and Quantum Mechanics
The 20th century witnessed a surge of discoveries related to subatomic particles, including positrons, muons, and neutrinos. The advent of quantum mechanics revolutionized our understanding of the behavior of electrons and other particles at the atomic and subatomic levels. Pioneering scientists like Werner Heisenberg, Erwin Schrödinger, and Paul Dirac contributed to the development of quantum mechanics, providing powerful mathematical tools to study electrons and other particles.
VIII. Electron in Modern Technology
The discovery of the electron not only transformed our understanding of fundamental physics but also had a profound impact on technology. The development of vacuum tubes, electron microscopes, transistors, and integrated circuits relied heavily on our understanding of electron behavior. These technological advancements revolutionized various industries and paved the way for the digital age.
Conclusion:
The discovery of the electron was a remarkable journey that involved the efforts of many brilliant minds throughout history. From the early observations of cathode rays to the precise measurements of the electron’s charge and mass, the quest to understand this subatomic particle reshaped the landscape of physics and technology. The electron’s discovery laid the groundwork for modern atomic theory, quantum mechanics, and countless technological innovations. As we continue to explore the mysteries of the universe, the legacy of the electron’s discovery reminds us of the power of human curiosity and the potential for profound scientific breakthroughs.




Pingback: Delhi University Review - Padho Hindi Me